第85页
- 第1页
- 第2页
- 第3页
- 第4页
- 第5页
- 第6页
- 第7页
- 第8页
- 第9页
- 第10页
- 第11页
- 第12页
- 第13页
- 第14页
- 第15页
- 第16页
- 第17页
- 第18页
- 第19页
- 第20页
- 第21页
- 第22页
- 第23页
- 第24页
- 第25页
- 第26页
- 第27页
- 第28页
- 第29页
- 第30页
- 第31页
- 第32页
- 第33页
- 第34页
- 第35页
- 第36页
- 第37页
- 第38页
- 第39页
- 第40页
- 第41页
- 第42页
- 第43页
- 第44页
- 第45页
- 第46页
- 第47页
- 第48页
- 第49页
- 第50页
- 第51页
- 第52页
- 第53页
- 第54页
- 第55页
- 第56页
- 第57页
- 第58页
- 第59页
- 第60页
- 第61页
- 第62页
- 第63页
- 第64页
- 第65页
- 第66页
- 第67页
- 第68页
- 第69页
- 第70页
- 第71页
- 第72页
- 第73页
- 第74页
- 第75页
- 第76页
- 第77页
- 第78页
- 第79页
- 第80页
- 第81页
- 第82页
- 第83页
- 第84页
- 第85页
- 第86页
- 第87页
- 第88页
- 第89页
- 第90页
- 第91页
- 第92页
- 第93页
- 第94页
- 第95页
- 第96页
- 第97页
- 第98页
- 第99页
- 第100页
- 第101页
- 第102页
- 第103页
- 第104页
- 第105页
- 第106页
- 第107页
- 第108页
- 第109页
- 第110页
- 第111页
- 第112页
- 第113页
- 第114页
- 第115页
- 第116页
- 第117页
- 第118页
- 第119页
- 第120页
- 第121页
- 第122页
- 第123页
- 第124页
- 第125页
- 第126页
- 第127页
- 第128页
- 第129页
- 第130页
15.某化学小组为了研究外界条件对化学反应速率的影响,进行了如下实验:
[实验原理]$2\text{KMnO}_{4} + 5\text{H}_{2}\text{C}_{2}\text{O}_{4} + 3\text{H}_{2}\text{SO}_{4}\xlongequal{}\text{K}_{2}\text{SO}_{4} + 2\text{MnSO}_{4} + 10\text{CO}_{2}\uparrow + 8\text{H}_{2}\text{O}$
[实验内容及记录]
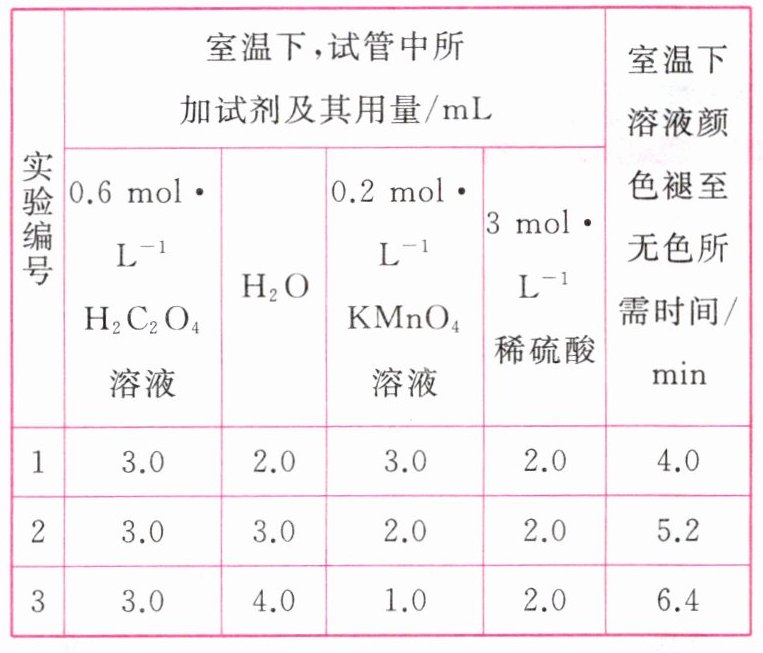
请回答下列问题:
(1)根据上表中的实验数据,可以得到的结论是____________________。
(2)利用实验1中数据计算,$v(\text{KMnO}_{4}) = $____________________。
(3)该化学小组同学根据经验绘制了$n(\text{Mn}^{2 + })$随时间变化趋势的示意图,如图甲所示。但有同学查阅已有的实验资料发现,该实验过程中$n(\text{Mn}^{2 + })$随时间变化的趋势应如图乙所示。该小组同学根据图乙所示信息提出了新的假设,并继续进行实验探究。
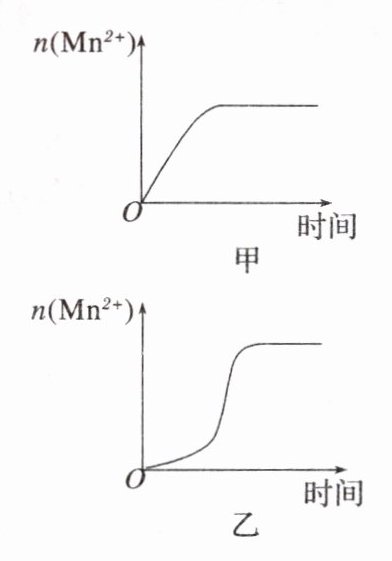
①该小组同学提出的假设是____________________。
②请你帮助该小组同学补全实验方案,并填写表中空白。
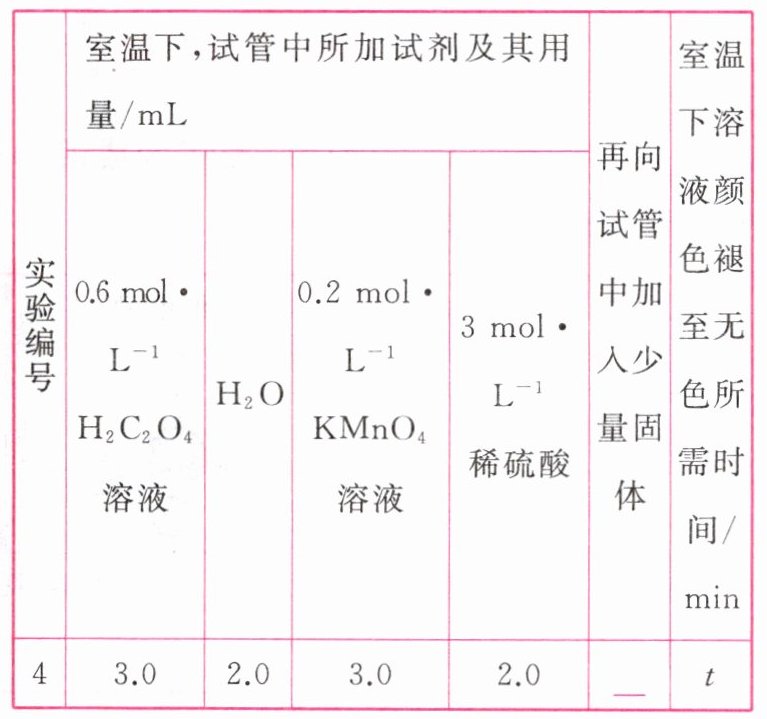
③若该小组同学提出的假设成立,应观察到的现象是____________________。
[实验原理]$2\text{KMnO}_{4} + 5\text{H}_{2}\text{C}_{2}\text{O}_{4} + 3\text{H}_{2}\text{SO}_{4}\xlongequal{}\text{K}_{2}\text{SO}_{4} + 2\text{MnSO}_{4} + 10\text{CO}_{2}\uparrow + 8\text{H}_{2}\text{O}$
[实验内容及记录]

请回答下列问题:
(1)根据上表中的实验数据,可以得到的结论是____________________。
(2)利用实验1中数据计算,$v(\text{KMnO}_{4}) = $____________________。
(3)该化学小组同学根据经验绘制了$n(\text{Mn}^{2 + })$随时间变化趋势的示意图,如图甲所示。但有同学查阅已有的实验资料发现,该实验过程中$n(\text{Mn}^{2 + })$随时间变化的趋势应如图乙所示。该小组同学根据图乙所示信息提出了新的假设,并继续进行实验探究。

①该小组同学提出的假设是____________________。
②请你帮助该小组同学补全实验方案,并填写表中空白。

③若该小组同学提出的假设成立,应观察到的现象是____________________。
答案:
答案 (1)其他条件相同时,增大KMnO₄溶液浓度(或反应物浓度),反应速率增大
(2)1.5×10⁻² mol·L⁻¹·min⁻¹
(3)①生成物中的MnSO₄为该反应的催化剂(或Mn²⁺对该反应有催化作用)
②MnSO₄
③与实验1比较,溶液褪色所需时间更短[或所用时间(t)小于4.0 min及其他合理答案]
<u>***Micro Judgment***</u>[](https://www.latexeditor.org/)
<ol>[](https://www.latexeditor.org/)
<li>If an organic compound contains carbon then all compounds containing carbon are organic compounds.[](https://www.latexeditor.org/)( )[](https://www.latexeditor.org/)</li>[](https://www.latexeditor.org/)
<li>All organic compounds are flammable.[](https://www.latexeditor.org/)( )[](https://www.latexeditor.org/)</li>[](https://www.latexeditor.org/)
<li>When an alkane burns completely in oxygen it produces only carbon dioxide and water.[](https://www.latexeditor.org/)( )[](https://www.latexeditor.org/)</li>[](https://www.latexeditor.org/)
<li>The reaction between methane and chlorine is a displacement reaction.[](https://www.latexeditor.org/)( )[](https://www.latexeditor.org/)</li>[](https://www.latexeditor.org/)
<li>The general formula for alkanes is $\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}$ ($ n≥≥≥≥≥≥≥≥≥≥≥≥ ≥ ≥ ≥ ≥ ≥ ≥ ≥ ≥ ≥ ≥ ≥ ≥ ≥ ≥ ≥ ≤ ≤ ≤ ≤ ≤ ≤ ≤ ≤ ≤ ≤ ≤ ≤ ≤ ≤≤≤≤≤≤≤≤≤≤≤≤≤≤≤), but not all compounds conforming to this formula are alkanes.[](https://www.latexeditor.org/)( )[](https://www.latexeditor.org/)</li>[](https://www.latexeditor.org/)
<li>A characteristic property of alkanes is their ability to undergo substitution reactions with halogen elements.[](https://www.latexeditor.org/)( )[](https://www.latexeditor.org/)</li>[](https://www.latexeditor.org/)
<li>Alkanes are stable in nature and do not react with acidic potassium permanganate solution.[](https://www.latexeditor.org/)( )[](https://www.latexeditor.org/)</li>[](https://www.latexeditor.org/)
<li>Isomers have the same elemental composition.< spanstyle =" color # FF FF FF " > [] </ span > () </ li > [] </ li > [] </ li > [] </ li > [] </ li > [] </ li > [] </ li > [] </ li > [] </ li > [] </ li > [] </ ol >< spanstyle =" color # FF FF FF " > [ end ] </ span >< u >< strong >< spanstyle =" color # FF FF FF " ></ strong ></ u >< u >< strong >< spanstyle =" color # FF FF FF " ></ strong ></ u >< u >< strong >< spanstyle =" color # FF FF FF " ></ strong ></ u >< u >< strong >< spanstyle =" color # FF FF FF " ></ strong ></ u >< u >< strong >< spanstyle =" color # FF FF FF " ></ strong ></ u >= >= >= >= >= >= >= >= >= >= >= == == == == == == == == == == === === === === === === === === === === === === === === === !== !== !== !== !== !== !== !==!==!==!==!==!==!==!==!==!==!==!=!=!=!=!=!=!=!=!=!=!=!=!=!!!!!!!!!!!!!!!!!!!!!*Micro Training*[]()[]()[]()[]()[]()[]()[]()[]()[]()[]()
<ol>[](https://www.latexeditor.org/)
<li>If an organic compound contains carbon then all compounds containing carbon are organic compounds.[](https://www.latexeditor.org/)( )[](https://www.latexeditor.org/)</li>[](https://www.latexeditor.org/)
<li>All organic compounds are flammable.[](https://www.latexeditor.org/)( )[](https://www.latexeditor.org/)</li>[](https://www.latexeditor.org/)
<li>When an alkane burns completely in oxygen it produces only carbon dioxide and water.[](https://www.latexeditor.org/)( )[](https://www.latexeditor.org/)</li>[](https://www.latexeditor.org/)
<li>The reaction between methane and chlorine is a displacement reaction.[](https://www.latexeditor.org/)( )[](https://www.latexeditor.org/)</li>[](https://www.latexeditor.org/)
<li>The general formula for alkanes is $\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}\mathrmsubstituent{n}}$ ($ n≥≥≥≥≥≥≥≥≥≥≥≥ ≥ ≥ ≥ ≥ ≥ ≥ ≥ ≥ ≥ ≥ ≥ ≥ ≥ ≥ ≥ ≤ ≤ ≤ ≤ ≤ ≤ ≤ ≤ ≤ ≤ ≤ ≤ ≤ ≤≤≤≤≤≤≤≤≤≤≤≤≤≤≤), but not all compounds conforming to this formula are alkanes.[](https://www.latexeditor.org/)( )[](https://www.latexeditor.org/)</li>[](https://www.latexeditor.org/)
<li>A characteristic property of alkanes is their ability to undergo substitution reactions with halogen elements.[](https://www.latexeditor.org/)( )[](https://www.latexeditor.org/)</li>[](https://www.latexeditor.org/)
<li>Alkanes are stable in nature and do not react with acidic potassium permanganate solution.[](https://www.latexeditor.org/)( )[](https://www.latexeditor.org/)</li>[](https://www.latexeditor.org/)
<li>Isomers have the same elemental composition.< spanstyle =" color # FF FF FF " > [] </ span > () </ li > [] </ li > [] </ li > [] </ li > [] </ li > [] </ li > [] </ li > [] </ li > [] </ li > [] </ li > [] </ ol >< spanstyle =" color # FF FF FF " > [ end ] </ span >< u >< strong >< spanstyle =" color # FF FF FF " ></ strong ></ u >< u >< strong >< spanstyle =" color # FF FF FF " ></ strong ></ u >< u >< strong >< spanstyle =" color # FF FF FF " ></ strong ></ u >< u >< strong >< spanstyle =" color # FF FF FF " ></ strong ></ u >< u >< strong >< spanstyle =" color # FF FF FF " ></ strong ></ u >= >= >= >= >= >= >= >= >= >= >= == == == == == == == == == == === === === === === === === === === === === === === === === !== !== !== !== !== !== !== !==!==!==!==!==!==!==!==!==!==!==!=!=!=!=!=!=!=!=!=!=!=!=!=!!!!!!!!!!!!!!!!!!!!!*Micro Training*[]()[]()[]()[]()[]()[]()[]()[]()[]()[]()
答案:
微判断:1.× 2.× 3.√ 4.× 5.× 6.√ 7.√ 8.√ 9.× 10.√ 11.× 12.×
<u>*Micro Training*</u>[][][][][][][][][][]()[][]()[][]()[][]()[][]()[][]()[][]()[][]()[][]()[][]()[][]()[][]()[][]()[][]()[][]()[][]()[][](). The following statement about alkanes is incorrect:< br />& nbsp;& nbsp;& nbsp;& nbsp;& nbsp;& nbsp;& nbsp;& nbsp;& nbsp;& nbsp;& nbsp;& nbsp;& nbsp;& nbsp;& nbsp;& nbsp;& nbsp;. Each carbon atom in an alkane forms four single covalent bonds<br /> nbsp. The four carbon atoms in n-butane can be on the same straight line<br /> . Methane ethane propane each have only one possible structure<br /> . No alkane molecule can have a planar structure<br />)<br />)[ end ]< br />)[ end ]< br />)[ end ]< br />)[ end ]< br />)[ end ]< br />)[ end ]< br />)[ end ]< br />)[ end ]
答案:
微训练:1.B 2.C
查看更多完整答案,请扫码查看


