2026年突唯中考中考总复习九年级化学全一册通用版河南专版
注:目前有些书本章节名称可能整理的还不是很完善,但都是按照顺序排列的,请同学们按照顺序仔细查找。练习册 2026年突唯中考中考总复习九年级化学全一册通用版河南专版 答案主要是用来给同学们做完题方便对答案用的,请勿直接抄袭。
第67页
- 第1页
- 第2页
- 第3页
- 第4页
- 第5页
- 第6页
- 第7页
- 第8页
- 第9页
- 第10页
- 第11页
- 第12页
- 第13页
- 第14页
- 第15页
- 第16页
- 第17页
- 第18页
- 第19页
- 第20页
- 第21页
- 第22页
- 第23页
- 第24页
- 第25页
- 第26页
- 第27页
- 第28页
- 第29页
- 第30页
- 第31页
- 第32页
- 第33页
- 第34页
- 第35页
- 第36页
- 第37页
- 第38页
- 第39页
- 第40页
- 第41页
- 第42页
- 第43页
- 第44页
- 第45页
- 第46页
- 第47页
- 第48页
- 第49页
- 第50页
- 第51页
- 第52页
- 第53页
- 第54页
- 第55页
- 第56页
- 第57页
- 第58页
- 第59页
- 第60页
- 第61页
- 第62页
- 第63页
- 第64页
- 第65页
- 第66页
- 第67页
- 第68页
- 第69页
- 第70页
- 第71页
- 第72页
- 第73页
- 第74页
- 第75页
- 第76页
- 第77页
- 第78页
- 第79页
- 第80页
- 第81页
- 第82页
- 第83页
- 第84页
- 第85页
- 第86页
- 第87页
- 第88页
- 第89页
- 第90页
- 第91页
- 第92页
- 第93页
- 第94页
- 第95页
- 第96页
- 第97页
- 第98页
- 第99页
- 第100页
- 第101页
- 第102页
- 第103页
- 第104页
- 第105页
- 第106页
- 第107页
- 第108页
- 第109页
- 第110页
- 第111页
- 第112页
- 第113页
- 第114页
- 第115页
- 第116页
- 第117页
一、常见酸($\boldsymbol{HCl}$、$\boldsymbol{{H_{2}SO_{4}}}$)的化学性质(“酸五条”)
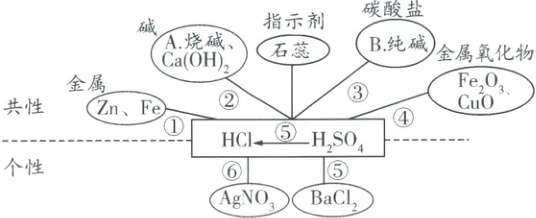
1. 请写出下列物质的化学式:A.
2. 请写出图中涉及反应的化学方程式
①_____、_____、_____、_____;
②_____、_____、_____、_____;
③_____、_____;
④_____、_____、_____、_____;
⑤_____;
⑥_____。

1. 请写出下列物质的化学式:A.
NaOH
$\boldsymbol{NaOH}$;B.Na₂CO₃
$\boldsymbol{{Na_{2}CO_{3}}}$。2. 请写出图中涉及反应的化学方程式
①_____、_____、_____、_____;
②_____、_____、_____、_____;
③_____、_____;
④_____、_____、_____、_____;
⑤_____;
⑥_____。
答案:
1. A:$\boldsymbol{NaOH}$;B:$\boldsymbol{{Na_{2}CO_{3}}}$。
2. ①$\boldsymbol{{Zn + 2HCl = ZnCl_{2} + H_{2}\uparrow}}$、$\boldsymbol{{Fe + 2HCl = FeCl_{2} + H_{2}\uparrow}}$、$\boldsymbol{{Zn + H_{2}SO_{4} = ZnSO_{4} + H_{2}\uparrow}}$、$\boldsymbol{{Fe + H_{2}SO_{4} = FeSO_{4} + H_{2}\uparrow}}$;
②$\boldsymbol{{HCl + NaOH = NaCl + H_{2}O}}$、$\boldsymbol{{H_{2}SO_{4} + 2NaOH = Na_{2}SO_{4} + 2H_{2}O}}$、$\boldsymbol{{2HCl + Ca(OH)_{2} = CaCl_{2} + 2H_{2}O}}$、$\boldsymbol{{H_{2}SO_{4} + Ca(OH)_{2} = CaSO_{4} + 2H_{2}O}}$;
③$\boldsymbol{{2HCl + Na_{2}CO_{3} = 2NaCl + H_{2}O + CO_{2}\uparrow}}$、$\boldsymbol{{H_{2}SO_{4} + Na_{2}CO_{3} = Na_{2}SO_{4} + H_{2}O + CO_{2}\uparrow}}$;
④$\boldsymbol{{6HCl + Fe_{2}O_{3} = 2FeCl_{3} + 3H_{2}O}}$、$\boldsymbol{{Fe_{2}O_{3} + 3H_{2}SO_{4} = Fe_{2}(SO_{4})_{3} + 3H_{2}O}}$、$\boldsymbol{{2HCl + CuO = CuCl_{2} + H_{2}O}}$、$\boldsymbol{{H_{2}SO_{4} + CuO = CuSO_{4} + H_{2}O}}$;
⑤$\boldsymbol{{H_{2}SO_{4} + BaCl_{2} = BaSO_{4}\downarrow + 2HCl}}$;
⑥$\boldsymbol{{HCl + AgNO_{3} = AgCl\downarrow + HNO_{3}}}$。
2. ①$\boldsymbol{{Zn + 2HCl = ZnCl_{2} + H_{2}\uparrow}}$、$\boldsymbol{{Fe + 2HCl = FeCl_{2} + H_{2}\uparrow}}$、$\boldsymbol{{Zn + H_{2}SO_{4} = ZnSO_{4} + H_{2}\uparrow}}$、$\boldsymbol{{Fe + H_{2}SO_{4} = FeSO_{4} + H_{2}\uparrow}}$;
②$\boldsymbol{{HCl + NaOH = NaCl + H_{2}O}}$、$\boldsymbol{{H_{2}SO_{4} + 2NaOH = Na_{2}SO_{4} + 2H_{2}O}}$、$\boldsymbol{{2HCl + Ca(OH)_{2} = CaCl_{2} + 2H_{2}O}}$、$\boldsymbol{{H_{2}SO_{4} + Ca(OH)_{2} = CaSO_{4} + 2H_{2}O}}$;
③$\boldsymbol{{2HCl + Na_{2}CO_{3} = 2NaCl + H_{2}O + CO_{2}\uparrow}}$、$\boldsymbol{{H_{2}SO_{4} + Na_{2}CO_{3} = Na_{2}SO_{4} + H_{2}O + CO_{2}\uparrow}}$;
④$\boldsymbol{{6HCl + Fe_{2}O_{3} = 2FeCl_{3} + 3H_{2}O}}$、$\boldsymbol{{Fe_{2}O_{3} + 3H_{2}SO_{4} = Fe_{2}(SO_{4})_{3} + 3H_{2}O}}$、$\boldsymbol{{2HCl + CuO = CuCl_{2} + H_{2}O}}$、$\boldsymbol{{H_{2}SO_{4} + CuO = CuSO_{4} + H_{2}O}}$;
⑤$\boldsymbol{{H_{2}SO_{4} + BaCl_{2} = BaSO_{4}\downarrow + 2HCl}}$;
⑥$\boldsymbol{{HCl + AgNO_{3} = AgCl\downarrow + HNO_{3}}}$。
3. 金属除锈的化学方程式为$\boldsymbol{{Fe_{2}O_{3} + 6HCl = 2FeCl_{3} + 3H_{2}O}}$(或$\boldsymbol{{Fe_{2}O_{3} + 3H_{2}SO_{4} = Fe_{2}(SO_{4})_{3} + 3H_{2}O}}$),生活中常用含${Mg(OH)_{2}}$的药物以及${NaHCO_{3}}$治疗胃酸过多症的化学方程式分别为$\boldsymbol{{Mg(OH)_{2} + 2HCl = MgCl_{2} + 2H_{2}O}}$、$\boldsymbol{{NaHCO_{3} + HCl = NaCl + H_{2}O + CO_{2}\uparrow}}$。
答案:
3.Fe₂O₃ + 6HCl = 2FeCl₃ + 3H₂O(合理即可)
Mg(OH)₂ + 2HCl = MgCl₂ + 2H₂O
NaHCO₃ + HCl = NaCl + H₂O + CO₂↑
Mg(OH)₂ + 2HCl = MgCl₂ + 2H₂O
NaHCO₃ + HCl = NaCl + H₂O + CO₂↑
二、常见碱[$\boldsymbol{NaOH}$、$\boldsymbol{{Ca(OH)_{2}}}$]的化学性质(“碱四条”)
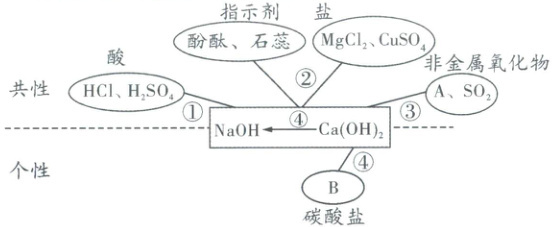
1. 请写出下列物质的化学式:A.
2. 请写出图中涉及反应的化学方程式

1. 请写出下列物质的化学式:A.
CO₂
$\boldsymbol{{CO_{2}}}$;B.Na₂CO₃(合理即可)
$\boldsymbol{{Na_{2}CO_{3}}}$。2. 请写出图中涉及反应的化学方程式

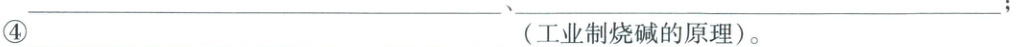
答案:
1. A:$\boldsymbol{CO_{2}}$;B:$\boldsymbol{Na_{2}CO_{3}}$(合理即可)。
2. ①$\boldsymbol{HCl + NaOH = NaCl + H_{2}O}$、$\boldsymbol{H_{2}SO_{4} + 2NaOH = Na_{2}SO_{4} + 2H_{2}O}$、$\boldsymbol{2HCl + Ca(OH)_{2} = CaCl_{2} + 2H_{2}O}$、$\boldsymbol{H_{2}SO_{4} + Ca(OH)_{2} = CaSO_{4} + 2H_{2}O}$;
②$\boldsymbol{2NaOH + MgCl_{2} = Mg(OH)_{2}\downarrow + 2NaCl}$、$\boldsymbol{2NaOH + CuSO_{4} = Cu(OH)_{2}\downarrow + Na_{2}SO_{4}}$、$\boldsymbol{Ca(OH)_{2} + MgCl_{2} = Mg(OH)_{2}\downarrow + CaCl_{2}}$、$\boldsymbol{Ca(OH)_{2} + CuSO_{4} = Cu(OH)_{2}\downarrow + CaSO_{4}}$;
③$\boldsymbol{2NaOH + CO_{2} = Na_{2}CO_{3} + H_{2}O}$、$\boldsymbol{2NaOH + SO_{2} = Na_{2}SO_{3} + H_{2}O}$、$\boldsymbol{Ca(OH)_{2} + CO_{2} = CaCO_{3}\downarrow + H_{2}O}$、$\boldsymbol{Ca(OH)_{2} + SO_{2} = CaSO_{3}\downarrow + H_{2}O}$;
④$\boldsymbol{Ca(OH)_{2} + Na_{2}CO_{3} = CaCO_{3}\downarrow + 2NaOH}$(工业制烧碱的原理)。
2. ①$\boldsymbol{HCl + NaOH = NaCl + H_{2}O}$、$\boldsymbol{H_{2}SO_{4} + 2NaOH = Na_{2}SO_{4} + 2H_{2}O}$、$\boldsymbol{2HCl + Ca(OH)_{2} = CaCl_{2} + 2H_{2}O}$、$\boldsymbol{H_{2}SO_{4} + Ca(OH)_{2} = CaSO_{4} + 2H_{2}O}$;
②$\boldsymbol{2NaOH + MgCl_{2} = Mg(OH)_{2}\downarrow + 2NaCl}$、$\boldsymbol{2NaOH + CuSO_{4} = Cu(OH)_{2}\downarrow + Na_{2}SO_{4}}$、$\boldsymbol{Ca(OH)_{2} + MgCl_{2} = Mg(OH)_{2}\downarrow + CaCl_{2}}$、$\boldsymbol{Ca(OH)_{2} + CuSO_{4} = Cu(OH)_{2}\downarrow + CaSO_{4}}$;
③$\boldsymbol{2NaOH + CO_{2} = Na_{2}CO_{3} + H_{2}O}$、$\boldsymbol{2NaOH + SO_{2} = Na_{2}SO_{3} + H_{2}O}$、$\boldsymbol{Ca(OH)_{2} + CO_{2} = CaCO_{3}\downarrow + H_{2}O}$、$\boldsymbol{Ca(OH)_{2} + SO_{2} = CaSO_{3}\downarrow + H_{2}O}$;
④$\boldsymbol{Ca(OH)_{2} + Na_{2}CO_{3} = CaCO_{3}\downarrow + 2NaOH}$(工业制烧碱的原理)。
查看更多完整答案,请扫码查看


